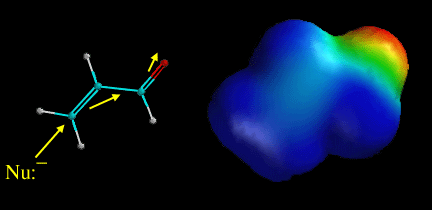
A) Alkenes adjacent to a carbonyl are conjugated and are therefore electrophilic.
B) These species are called a,b unsaturated carbonyl compounds.
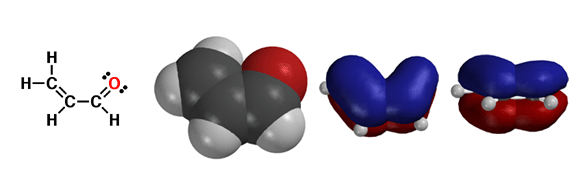
C) a,b unsaturated carbonyl compounds are conjugated, in that the pi electrons of the C=C and C=O bonds can delocalize over all four atoms. This lends some degree of extra stabilization to these species, because pi electrons prefer to delocalize.
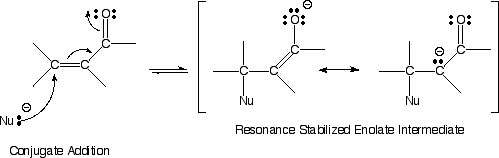
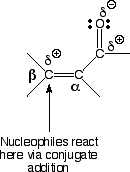
D) Nucleophiles can, however, react at the b carbon atom in a process called conjugate addition.
E) Conjugate addition is favorable because the intermediate formed is a resonance stabilized enolate, thus relatively low energy.